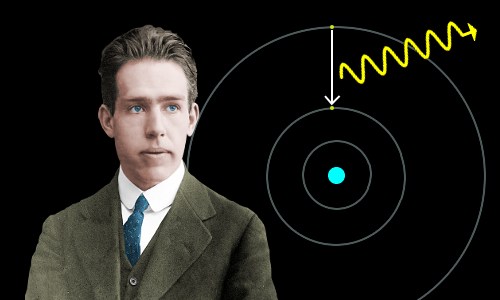
Bohr model description of the structure of atoms especially that of hydrogen proposed 1913 by the danish physicist niels bohr the bohr model of the atom a radical departure from earlier classical descriptions was the first that incorporated quantum theory and was the predecessor of wholly quantum mechanical models.
Niels bohr sheet of metal experiment.
Ab lagrelius westphal via american institute of physics.
So think of the model as a spherical christmas cake.
7 october 1885 18 november 1962 was a danish physicist who made foundational contributions to understanding atomic structure and quantum theory for which he received the nobel prize in physics in 1922.
During the early 20th century new zealand scientist ernest rutherford conducted an experiment.
The bohr model and all of its successors describe the properties of.
Bohr developed the bohr model of the atom in which he proposed.
Bright red light which statement describes a major drawback of the bohr model that caused scientists to replace it.
Bohr model of the atom is rooted in quantum mechanics.
The danish physicist niels bohr in 1911 bohr completed his education at the university of copenhagen when he was awarded a doctor of philosophy ph d in physics for his work on the behavior of.
A 1963 danish stamp honored bohr on the 50th anniversary of his atomic theory.
In an experiment shining which type of light on a strip of metal would be least likely to produce the photoelectric effect.
Thomson s plum pudding model viewed the atom as a massive blob of positive charge dotted with negative charges.
A plum pudding was a christmas cake studded with raisins plums.
Albert einstein introduced the photoelectric effect in 1905 where electrons would be emitted when light of a certain frequency shines on a metal.
He shot electrically charged alpha particles at thin gold foil.
These discoveries were later credited by niels bohr when creating the bohr model.
Bohr was also a philosopher and a promoter of scientific research.
Bohr nailed the true structure of an atom in 1913.
This theory was postulated by a danish physicist named neil bohr in 1922 and has got its name as bohr atomic model.
When rutherford shot α particles through gold foil he found.
Rutherford s experiment showed that the atom does not contain a uniform distribution of charge.
After finding his work at odds with thomson s bohr joined the manchester university lab of ernest rutherford who had also studied under thomson.
This experiment helped rutherford and danish scientist niels bohr develop a way of thinking about the structure of an atom.